Revaree Reviews
If you’re looking for relief from vaginal dryness, these hyaluronic acid suppositories may be able to help.

Photo by Innerbody Research
During the menopausal transition, your body goes through a host of changes, the most far-reaching of which is decreased estrogen production. The lower levels of this hormone can lead to a wide range of symptoms, including vaginal dryness.1 Despite being one of the most common symptoms of menopause — the estimated prevalence is up to 60% of menopausal women — many patients don’t report this symptom due to factors like embarrassment,2 3 being unaware of available treatments, or having a dismissive healthcare provider.4
The good news is that there are a number of vaginal dryness remedies on the market, many of which can be obtained discreetly online without a prescription. One of these options is Revaree from Bonafide Health. The company states its hyaluronic acid vaginal suppositories can restore “your body’s natural moisture” to bring you relief in “as little as nine days.” But are these claims legitimate?
In our review of Revaree, we analyze the research behind the product along with its safety, cost, convenience, and everything in between to help you decide if it’s the right option for you.
Our Findings
Revaree is a clinically studied, hormone-free hyaluronic acid vaginal insert that should be an effective and generally safe option for adults seeking relief from vaginal dryness due to menopause, medications, cancer treatment, and more. Research suggests the efficacy of hyaluronic acid for vaginal dryness is equivalent to estrogen, “the gold standard” treatment. It’s important to know that Revaree is not compatible with condoms, and since some experts recommend avoiding natural oils for vaginal dryness — like the sweet almond oil in Revaree Plus — we suggest sticking with the original formula.
Pros
- Studies have found hyaluronic acid to be an effective remedy for vaginal dryness
- Research specifically on Revaree has had positive outcomes
- Both strengths are FDA-cleared as Class II medical devices
- Adheres to Good Manufacturing Practices
- Third-party tested for identity, purity, potency, allergens, and more
- Suppositories are fairly small
- The box your order comes in is discreet
- HSA/FSA cards are accepted at checkout (depending on your plan)
- Subscriptions can net you up to a 32% discount
Cons
- Not compatible with latex, polyurethane, or polyisoprene condoms
- Revaree Plus contains almond oil, which some experts caution against
- Rarely may lead to UTIs, bacterial vaginosis (BV), or yeast infections
- More expensive per dose/use than some competing products
- May take up to a couple of months to work, depending on dryness severity
Purchase options
Revaree is available from Bonafide’s website as well as the company’s store on Amazon. One-time purchases are a bit pricey in either location, but subscriptions bring those costs down substantially — especially when you buy directly from Bonafide. And if you’re not quite sure about committing to a full order of Revaree, the Bonafide website offers a nine-day supply trial for only a $5 shipping fee. Ultimately, subscribing is the winning path, and your best value is subscribing on the Bonafide website itself.
Jump to:
Why you should trust us
Over the past two decades, Innerbody Research has helped tens of millions of readers make more informed decisions involving staying healthy and living healthier lifestyles.
For our review of Revaree, we spent over 100 hours researching the safety and efficacy of hyaluronic acid, estrogen, and other treatments for vaginal dryness and symptoms of the genitourinary syndrome of menopause (GSM). We also bought Revaree, communicated with customer service, and investigated common user complaints to bring you all the details you need to make the purchasing decisions best suited for your health.
Additionally, like all health-related content on this website, this review was thoroughly vetted by one or more members of our Medical Review Board for accuracy. As more research on treatments for GSM is conducted, we’ll update this review accordingly.
How we evaluated Revaree
To evaluate Revaree, we considered four essential customer-centric criteria: safety, efficacy, cost, and convenience. Overall, Revaree performed very well, but it did earn higher marks in some categories (safety and efficacy) than others (cost and convenience).
Let’s go over the details of each criterion.
Safety
Based on the data from multiple studies and systematic reviews, Revaree should be a generally safe option for most adults seeking relief from vaginal dryness. The only people explicitly told to be cautious in the Revaree patient information booklet are those who are pregnant or nursing, as Revaree use has not been studied in those populations. Though it’s recommended that everyone ask a doctor before using Revaree, medical advice before use is particularly important for pregnant or breastfeeding individuals.
The body naturally produces hyaluronic acid to lubricate joints, maintain hydration, promote skin flexibility, and support wound healing.5 It’s considered safe to use by experts, and it can be found in supplements, skin care products, eye drops, some prescription medications, and various vaginal dryness remedies. In most clinical research on hyaluronic acid for vaginal dryness, researchers note that it’s a safe ingredient and that no serious adverse events related to the treatment were experienced by subjects.6 Occasionally, patients have reported mild side effects like vaginal irritation or discomfort, but they typically subsided with continued use of the product.7
A 2013 trial investigating hyaluronic acid gel for vaginal dryness mentioned that, out of 72 subjects in the test group, three developed a yeast infection and one developed bacterial vaginosis (BV).8 In rare cases, Revaree may lead to UTIs, which, if left untreated, can become dangerous.9
While these side effects are uncommon, it’s recommended you speak to your doctor as soon as possible if you experience any new or worsening symptoms after starting Revaree.
Finally, we suggest either avoiding Revaree Plus — the extra-strength variety — or proceeding with caution before using it. This product contains sweet almond oil, which some experts recommend against for safety reasons.10 11 You also shouldn’t use Revaree Plus if you’re allergic to almonds or other tree nuts.
For further safety information, we go into more detail under the “Is Revaree safe?” section.
Effectiveness
Studies on both Revaree and hyaluronic acid for vaginal dryness in general have all seen positive outcomes.
As stated by the authors of a 2020 overview of the genitourinary syndrome of menopause (GSM), “Hormonal therapy with local estrogen products is generally considered the ‘gold standard’.”12 But, much of the research on treatments for GSM symptoms has found hyaluronic acid’s performance to be comparable to estrogen, often noting the differences between the two aren’t statistically significant.13 Hyaluronic acid — in suppository, cream, or gel form — is frequently dubbed an effective, safe, and well-tolerated option for vaginal dryness and other GSM symptoms, particularly in those who either can’t or don’t want to use vaginal estrogen therapy.14 15 16
Each treatment may have unique benefits, however. One study found that hyaluronic acid may be superior at improving symptoms of urinary incontinence,13 while another found estrogen could be the better option for those having issues with vaginal pH or dyspareunia (painful sexual intercourse).6 19 Of course, this is only based on the results of two studies, and more research is needed to say one is better than the other at specific symptom relief, but it’s still worth mentioning.
Data on Revaree had similar findings, with a survey,17 clinical trial,7 and study all noting that the majority of patients experienced relief from dryness and improvement in other symptoms, such as irritation, pain, and dyspareunia, after using the suppositories.18
For more information on the efficacy of Revaree, we delve deeper into the science under the “Does Revaree work?” section.
Cost
One-time purchases of Revaree or Revaree Plus are more expensive than some similar products, but opting for a subscription brings the costs down quite a bit. To illustrate this, the chart below compares the prices of Revaree and Revaree Plus to the hyaluronic acid suppositories from competitors Evvy and Neycher.
| Revaree | Revaree Plus | Evvy | Neycher | |
|---|---|---|---|---|
| Inserts per box | 10 | 10 | 12 | 10 |
| One-time purchase price | $65.00 | $65.00 | $59.00 | $46.95 |
| One-time price per dose | $6.50 | $6.50 | $4.92 | $4.70 |
| Price when you subscribe | $48 (monthly); $129 (3-month) | $52 (monthly); $141 (3-month) | N/A | $36.60 |
| Price per dose when you subscribe | $4.80 (monthly); $4.30 (3-month) | $5.20 (monthly); $4.70 (3-month) | N/A | $3.66 |
| HSA/FSA eligible? | ||||
| Extra notes | One-time purchases add $5 shipping; subscriptions ship free | One-time purchases add $5 shipping; subscriptions ship free | No subscriptions available; shipping is free | Ships free in the U.S. |
As you can see, a subscription to Revaree (which you can cancel at any time) brings the prices down to about the same or lower than the competition’s one-time purchase costs.
You can also save some money on Revaree by signing up for Bonafide Health’s rewards program. After registering your account, you can earn points on every order (one point per dollar spent) from Bonafide toward coupons for a discount ($5-$45 off) on a future purchase.
Convenience
Where Revaree may fall flat for certain people is its convenience (or occasional lack thereof). For many users, there won’t be any convenience concerns, but there are definitely some aspects of the product that could prove frustrating for some.
First of all, there are only ten suppositories per pack of Revaree or Revaree Plus. The patient guide says to use one insert every two or three days for 30 days. If you use one every three days, then you’ll be fine, but those with worse dryness who may need to use it every two days will be short five suppositories by the end of the 30-day cycle. We hope Bonafide Health will eventually offer a 15-count option, or even a smaller five-count optional add-on, for those who need more of them.
Next, a complaint we came across fairly frequently during our research was customers having issues using the suppositories, particularly due to the lack of an applicator. Several people stated that they needed to purchase a third-party applicator from Amazon. And, as another potential inconvenience, Bonafide Health specifically instructs customers to use Revaree right before bedtime, as you need to remain lying down for at least an hour or so to ensure the suppository melts and absorbs properly. In contrast, topicals, like the Vulva Coco Cream from competitor Wisp, can be used at any time of day without restriction.
Finally, it’s important to know that Revaree and Revaree Plus (and any other similar product containing glyceride or oil-based ingredients) are not condom safe.
Now, pivoting to some more positive aspects, we can report that Bonafide’s customer service was responsive and friendly, Revaree subscriptions are convenient and can be modified at any time, and the suppositories are quite small.

Photo by Innerbody Research
As pictured above, each suppository is slightly less than one inch long and about a half-inch wide. (These measurements are useful to know if you need to shop for a third-party applicator.)
What is Revaree?
Revaree — also known as “Repagyn” outside of the U.S. — is a hyaluronic acid vaginal insert sold by Bonafide Health (often shortened to just “Bonafide”), a women’s health supplement company based in New York.
Revaree is intended to help relieve bothersome vaginal dryness and the pain that often comes with it. In fact, research shows that vulvovaginal symptoms, like vaginal dryness, can lead to a decrease in quality of life comparable to that of chronic obstructive pulmonary disease, arthritis, and other chronic health conditions.20
Bonafide offers two varieties of this suppository: Revaree and Revaree Plus, the latter of which is an extra-strength version. Unlike similar vaginal dryness suppositories from competitors like Evvy and Neycher, the original-strength Revaree only contains hyaluronic acid as the key moisturizing ingredient. Other products — including Revaree Plus — tend to have additional inclusions, such as fatty acids, oils, vitamins, and so on. These other ingredients aren’t bad, per se, but some experts recommend against certain ones, like natural oils, for vaginal use.10 11
Bonafide Health’s company reputation
When investigating a company’s reputation, we usually look for a Better Business Bureau (BBB) page and a Trustpilot one. Unfortunately, Bonafide Health doesn’t have a Trustpilot page at the time of this review, but it does have a BBB profile.
Though the company isn’t a BBB Accredited Business, it has an A- rating, mainly due to the speed at which the company addresses customer complaints. On that note, there are 11 complaints and only 12 customer reviews (averaging out to a 1.17-star rating). The feedback is mainly negative, and the chief complaints include difficulty managing subscriptions, not receiving ordered products, bothersome side effects, or a lack of noticeable differences in symptoms.
Additionally, the BBB notes that, in 2023, it “wrote to Bonafide Health, LLC,” asking the company “to substantiate” various advertising claims for its products but never received a response.
There are a decent number of customer reviews on the Revaree product page, but we can’t know for sure whether or not they’re being filtered or if customers are being offered an incentive — like a coupon or reward points — for reviewing. On a positive note, Shop Pay (the checkout service Bonafide’s website uses) has verified most of the reviewers as actual customers, and there are at least some critical reviews awarding just 1-3 stars.
The cumulative score on Revaree’s product page is 4.5 stars based on over 3,200 reviews, and roughly 70% of those are 5-star ratings. While the positive reviews generally mention Revaree working well and reducing feelings of dryness and itching, several negative ones either mention difficulty using the product due to the lack of an applicator or express frustration at the financial investment needed to use it long-term. More rarely, some complaints note that not all of the melted suppository gets absorbed, leading to a bit of a mess in the morning.
So, while the reviews on Bonafide’s website contain some valuable information, we would still prefer the data to come from more trustworthy sources, like the BBB and Trustpilot. And since the company’s BBB page only contains a very small amount of feedback, it’s difficult to judge the entire business based on it alone.
Who is Revaree for?
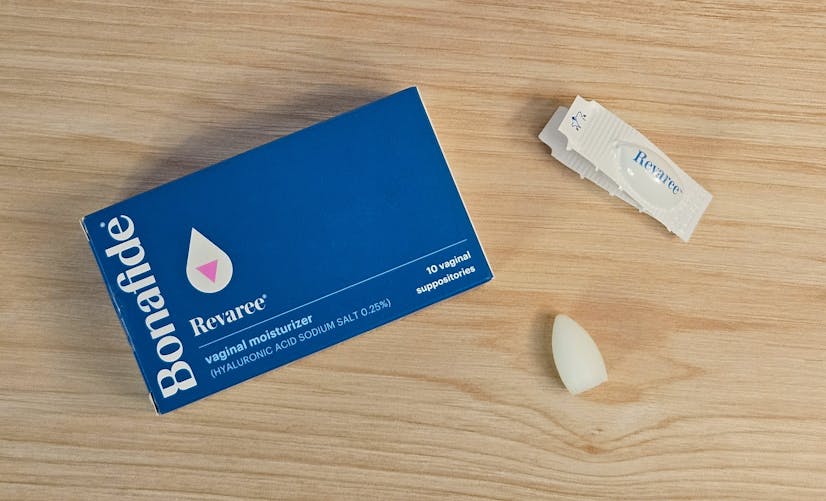
Photo by Innerbody Research
Though menopause is one of the most common reasons for vaginal dryness, it’s far from the only cause. In fact, an estimated 15% of premenopausal people experience it as well.21 Therefore, Revaree could be a good option for assigned female at birth (AFAB) individuals suffering from vaginal dryness due to:22
- Genitourinary syndrome of menopause (GSM), previously called “vaginal atrophy”
- Diabetes-related neuropathy or vascular impairment23
- An oophorectomy (surgical removal of ovaries)
- A hysterectomy (surgical removal of the uterus)24
- Cancer treatment
- Hormonal birth control
- Testosterone gender-affirming hormone therapy (GAHT)25
- Certain medications, like some antidepressants or antihistamines
- Sjogren’s syndrome26
- Rheumatoid arthritis26
- Other rheumatic autoimmune diseases26
Moreover, Revaree could be ideal for those seeking non-hormonal options. Research suggests hyaluronic acid — the main ingredient in Revaree suppositories — works as effectively as estrogen treatments for vaginal dryness.3
Who should look elsewhere?
While Revaree could be worth trying for many people, some groups may be better off looking elsewhere. The following list breaks down a few of them:
- If you’re pregnant or breastfeeding, it’s recommended that you either avoid Revaree or consult with your doctor first. As noted in the Revaree patient information booklet, no studies have been done to verify its safety in those populations.
- For people experiencing vaginal dryness or pain due to a yeast infection, antifungal medications or boric acid suppositories could be solutions.
- If you have vaginismus (a condition in which the vagina muscles involuntarily tense up or spasm when you try to insert something into it), then a topical treatment may be better than a suppository.
Is Revaree safe?
Revaree should be generally safe for most adult AFAB people experiencing vaginal dryness. It adheres to Good Manufacturing Practices (GMP), the products are third-party tested, and both Revaree and Revaree Plus are FDA-cleared as Class II medical devices.27 28 This FDA clearance means the agency considers them to be “safe and effective.”29
When it comes to Revaree’s star ingredient, hyaluronic acid, safety is generally high. After all, it’s a hydrating substance naturally produced by your body. As noted by the Cleveland Clinic, “Reactions or adverse effects from hyaluronic acid are rare.” But while the Cleveland Clinic also mentions that hyaluronic acid should be safe for pregnant or nursing individuals, the use of Revaree has not been tested in those groups, so getting a doctor’s opinion first is important.5
In most studies on hyaluronic acid for vaginal dryness, researchers note that patients reported no serious adverse effects. For example:
- A 2024 study funded by Bonafide Health comparing Revaree to vaginal estrogen for GSM symptoms found that “no treatment-related serious adverse events occurred in either study group.”7
- A 2008 clinical trial investigating vaginal suppositories containing hyaluronic acid, vitamin E, and vitamin A for GSM mentioned there were no adverse effects reported during treatment, and “a high level of compliance was registered.”15
- Researchers of a 2020 study “evaluating the effectiveness of hyaluronate-based” suppositories for GSM noted that “no severe adverse events were reported.”14
- A 2021 systematic review on hyaluronic acid for GSM in postmenopausal women stated that the compound “has a profile of efficacy, safety, and tolerability.”16
Though most studies don’t go into detail about side effects, a 2013 placebo-controlled clinical trial evaluating the efficacy and safety of a hyaluronic acid gel for vaginal dryness in 144 subjects (72 in the placebo group and 72 in the treatment one) offers some information. In total, there were 13 adverse events during the trial, seven of which were in the hyaluronic acid group. Researchers noted that only four of those events were found to be related to the test product: three experienced vulvovaginal candidiasis (a yeast infection), and one developed bacterial vaginosis (BV).8
Outside of scientific studies, the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database currently contains two separate adverse event reports on Revaree.
- The first one, from 2021, is a brief report from a 62-year-old customer who experienced burning from the product and discontinued use.30
- The second one, from 2023, is a more lengthy report from a physician detailing how a 59-year-old patient developed a vaginal infection along with “a urinary tract infection, a kidney infection, and near-sepsis” after restarting Revaree for four days following a short break. The patient was given IV fluids and antibiotics and then sent home with a two-week course of doxycycline.31
It’s important to point out that the physician who wrote the second report doesn’t outright claim Revaree caused the patient’s infections; rather, they state that it’s “impossible to rule out a possible contribution.” The physician goes on to mention that “UTIs, typically non-serious, have been reported in women taking the product.”31 If Revaree caused a UTI in the patient that was then left untreated, it could have spread to the kidneys and led to the patient’s urgent situation.9
All of this is to say that if you use Revaree and develop new or worsening symptoms, it’s very important to discontinue use and contact your doctor as soon as possible.
Revaree Plus safety (extra-strength formula)
Based on our research, we recommend sticking with Revaree's original strength. The extra-strength Revaree Plus contains both hyaluronic acid and sweet almond oil. Besides being a danger to those with allergies to almonds or other tree nuts, almond oil may not be the safest ingredient to use for vaginal dryness. For example, Cleveland Clinic OB-GYN Dr. Swapna Kollikonda, MD, recommends against natural oils, stating that “natural doesn’t always mean safer or better.”10
Additionally, Dr. Cynthia Abraham, a gynecologist and assistant professor in the Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, explains that oil-based products for vaginal dryness “can cause irritation and make condoms less effective.”11
Does Revaree work?
The existing research published about hyaluronic acid as a treatment for vaginal dryness suggests it should be an effective option for many people to find relief. Studies on the ingredient have been largely positive, with many stating the difference in performance between hyaluronic acid and estrogen — the “gold standard” treatment — isn’t statistically significant.12 13 This similar performance, particularly in menopausal women, may be due to the fact that lower estrogen levels in the urogenital tissue can lead to, among other things, a “decreased concentration of collagen, elastin, and hyaluronic acid.”12 So both estrogen and hyaluronic acid treatments may lead to similar replenishment of depleted vaginal hyaluronic acid levels.

Photo by Innerbody Research
Interestingly, the two treatments do seem to be slightly better at improving different symptoms. For example, a 2016 clinical trial comparing hyaluronic acid cream to estrogen for vaginal atrophy (GSM) found that only the former improved urinary incontinence. Both improved other symptoms, but the overall performance of hyaluronic acid was better than estrogen.13 Conversely, in a 2023 review, researchers mentioned that estrogen was superior in improving vaginal pH and relieving symptoms like dyspareunia, but they clarified that “the therapeutic efficacy of [hyaluronic acid] seems to be comparable to estrogen” and that it “can be used as an alternative to estrogen in patients who do not want to use estrogen.”6
As for studies on Revaree itself, there’s one comparing Revaree to a vaginal estrogen cream, one investigating the effects of Revaree Plus on those with moderate-to-severe vaginal dryness, and one national survey on patient satisfaction with Revaree.
The first one published was the national survey results. An optional survey was completed by 3,080 patients who’d been using Revaree for one month to over a year. In an effort to increase enrollment after the first nine months, patients were offered compensation of a $5 gift card (which could potentially have led to bias).17
Overall, 83% of respondents stated that Revaree reduced vaginal dryness, 77% noted reduced irritation, 69% reported more comfortable sexual intercourse, and 72% said Revaree improved their quality of life. In terms of how long it took the suppositories to work, 58% said it worked within two weeks, while 85% reported it took around eight weeks.17
In July of 2024, the results of Revaree’s clinical trial pitting it against a vaginal estrogen cream were published. Forty-five patients with GSM were given 12 weeks of either Revaree suppositories or vaginal estrogen cream. Researchers found that there were “no clinically meaningful differences between vaginal HLA [hyaluronic acid] and vaginal estrogen for the treatment of GSM.” Both options reduced symptoms like vaginal dryness, itching, and pain.7
The results of the Revaree Plus study were published in September of 2024. In this study, 70 patients with self-reported moderate-to-severe vaginal dryness used Revaree Plus twice a week for a month. After one week, over 50% of subjects reported an improvement. This increased to around 80% of subjects by the end of the study period.18
While all of the research specifically focused on Revaree can be valuable, the clinical trial stands out as the best piece of data. The survey information is interesting but the inclusion of compensation could skew the results. And the Revaree Plus study was both short and used an open-label design, the latter of which means that patients knew what product was being tested.
Ultimately, though, the data suggests that Revaree has the potential to help a large number of people suffering from vaginal dryness due to a variety of causes.
Revaree pricing, subscriptions, and returns
Compared to competing products — such as the hyaluronic acid suppositories from Evvy and Neycher — Revaree and Revaree Plus are a bit on the expensive side, but subscriptions can make them both much more affordable. Here’s how the costs work out:
| Revaree | Revaree Plus | |
|---|---|---|
| Inserts per box | 10 | 10 |
| One-time purchase price | $65.00 | $65.00 |
| One-time price per dose | $6.50 | $6.50 |
| Monthly subscription price | $48.00 | $52.00 |
| Monthly subscription price per dose | $4.80 | $5.20 |
| 3-month subscription price | $129.00 | $141.00 |
| 3-month subscription price per dose | $4.30 | $4.70 |
| HSA/FSA eligible? |
One-time purchases add a $5 shipping fee, while the subscriptions ship for free. This means that one-time purchases are even more expensive in the end. Since subscriptions can be modified (or canceled) at any time, it could be worthwhile to opt for the monthly subscription plan and then pause or reactivate it as needed.
If you aren’t sure about Revaree, Bonafide Health offers a nine-day supply trial for only the cost of shipping ($5). Do keep in mind that signing up for this trial also signs you up for a monthly subscription that will activate — and charge you $48 — after 14 days.
As with many companies that sell health and wellness products, Bonafide Health has a strict return policy. Only unopened, unused products can be returned or exchanged within 30 days of order shipment (this time is reduced to 14 days for subsequent or refill orders).
Alternatives to Revaree
Though Revaree is a promising remedy for vaginal dryness, it’s far from the only option out there. Below, we cover a few alternative options, including other hyaluronic acid products, hormone therapies, and topicals.
Other hyaluronic acid suppositories
If the idea of a hyaluronic acid suppository sounds ideal, but you aren’t sold on Revaree, then the options from competitors Evvy and Neycher may be worth considering.
Unlike Bonafide Health, Evvy is a telehealth platform, so this option could be ideal for those who would like a doctor to be directly involved in their treatment.
Each pack of Evvy’s Hyaluronic Acid Suppositories contains 12 inserts for $59 ($4.92 each). Though this is less expensive than a single purchase of Revaree, it requires a prescription for purchase, and there are no subscription discounts available. The need for a prescription from Evvy is interesting because there aren’t any hormones or medications in its suppositories. They each contain a “moisturizing base of fatty acids” — containing coconut, corn, soy, and tree nut allergens — and 5mg of hyaluronic acid.
Neycher’s clinically tested Vaginal Moisturizer suppositories contain 10mg of hyaluronic acid along with vitamin A, vitamin E, tea tree oil, and the company’s proprietary “Regevag Blend.” Tea tree oil may be able to help combat yeast infections, according to cell study results, but more research is needed.32 And — as with the sweet almond oil in Revaree Plus — tea tree oil and other natural oils could be irritating and may not be safe for vaginal use.10 11
A one-time purchase for a single box of Neycher’s hyaluronic acid vaginal inserts is $46.95 ($4.70 each; ten suppositories). Subscriptions take 24% off, making the monthly price $36.60 ($3.66 each).
It’s important to note that, just like Revaree, neither of the above two options is compatible with condoms due to their glyceride or oil contents.
Non-hormonal creams and gels
For those who’d rather not use a suppository — or for those who can’t use one — there are gel and cream options available, too. Some products on the market include:
- Hyalo Gyn Vaginal Hydrating Gel: Each $30 hyaluronic acid gel comes with ten single-use vaginal applicators. According to the manufacturer, it’s only compatible with polyisoprene condoms.
- Wisp Vulva Coco Cream: This $25 topical coconut oil, jojoba oil, and aloe vera cream from the telehealth platform Wisp is available without a prescription. In contrast to Hyalo Gyn, it’s for external use only. The various oils in this product could break down condoms.
- Stripes Beauty Vag of Honor: Similar to Wisp’s cream, this $50 plant-based hyaluronic acid topical is for external use. The squalane (oil) content in this topical means it could degrade condoms.
Vaginal estrogen cream
If your vaginal dryness is more severe, vaginal estrogen treatment may be the way to go. Although a 2002 Women’s Health Initiative study linked hormone replacement therapy (HRT) to a higher risk of blood clots, stroke, and breast cancer, many experts now see that study as flawed.33 Of course, this isn’t to say HRT doesn’t come with risks, but localized, low-dose vaginal estrogen is not the same as oral systemic estrogen.
The authors of a 2019 review on vaginal estrogen use and chronic disease risk explained that the treatment “was not associated with a higher risk of cardiovascular disease or cancer” and that it can be “a highly effective treatment for genitourinary syndrome of menopause.” (As a side note, the researchers included heart attack, stroke, pulmonary embolism, and deep vein thrombosis under the umbrella of “cardiovascular disease.”)34
That being said, a few notable telehealth options for getting prescription vaginal estrogen therapy include:
- Winona: Touted as being “bioidentical,” this plant-based vaginal estrogen treatment cream is available for $89 per month. Though the company doesn’t state anything about it, the oils in the inactive ingredients likely make this product not condom-safe. HSA/FSA is accepted at checkout.
- Wisp: This 0.01% estradiol vaginal cream ($65 one time or $60 as a quarterly subscription) is available as a free delivery or local pharmacy pickup. HSA/FSA payments are accepted. According to the product page, the oil base makes this cream incompatible with latex condoms.
- Alloy: Only available as a three-month supply ($39.99 per month, or about $120 total, plus a $49 one-time consultation fee), this plant-based 0.01% estradiol vaginal cream is HSA/FSA eligible. The inactive ingredients aren’t listed, so its condom compatibility is unclear.
For more information, check out our comprehensive reviews of Winona and Wisp.
Osphena (non-hormonal prescription)
Some people may require a prescription-strength vaginal dryness remedy but prefer — or need — to avoid using estrogen therapy. Currently, the only FDA-approved non-hormonal option for the treatment of vaginal dryness and/or painful sexual intercourse due to GSM is a drug called ospemifene, sold under the brand name “Osphena.” As of the writing of this review, there are no generic options on the market.35
Clinical trials on ospemifene found the drug to be “highly effective” in relieving “the most bothersome symptoms of dyspareunia and vaginal dryness.” Moreover, the long-term safety data shows that the most common side effects seen in the trials were mild-to-moderate vasomotor symptoms, like hot flashes or night sweats, while the incidence of cardiovascular events was similar to placebo.36
Revaree FAQ
Sources
Innerbody uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy.
Turek, J., & Gąsior, Ł. (2023). Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders. Pharmacological Reports, 75(1), 32-43.
Cleveland Clinic. (2024). Menopause. Cleveland Clinic.
Naumova, I., & Castelo-Branco, C. (2018). Current treatment options for postmenopausal vaginal atrophy. International Journal of Women's Health, 10, 387-395.
Kingsberg, S. A., Schaffir, J., Faught, B. M., Pinkerton, J. V., Parish, S. J., Iglesia, C. B., Gudeman, J., Krop, J., & Simon, J. A. (2019). Female sexual health: Barriers to optimal outcomes and a roadmap for improved patient–clinician communications. Journal of Women's Health, 28(4), 432-443.
Cleveland Clinic. (2022). Hyaluronic acid. Cleveland Clinic.
Albalawi, N. S., Almohammadi, M. A., & Albalawi, A. R. (2023). Comparison of the efficacy of vaginal hyaluronic acid to estrogen for the treatment of vaginal atrophy in postmenopausal women: A systematic review. Cureus, 15(8), e44191.
Agrawal, S., LaPier, Z., Nagpal, S., Oot, A., Friedman, S., Hade, E. M., Nachtigall, L., Brucker, B. M., & Escobar, C. (2024). A randomized, pilot trial comparing vaginal hyaluronic acid to vaginal estrogen for the treatment of genitourinary syndrome of menopause. Menopause (New York, N.y.), 31(9), 750-755.
Chen, J., Geng, L., Song, X., Li, H., Giordan, N., & Liao, Q. (2013). Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: A multicenter, randomized, controlled, open‐label, parallel‐group, clinical trial. The Journal of Sexual Medicine, 10(6), 1575-1584.
Cleveland Clinic. (2023). Urinary tract infections. Cleveland Clinic.
Cleveland Clinic. (2022). What works for vaginal dryness, and is natural best? Cleveland Clinic Health Essentials.
Abraham, C. (2024). Experiencing vaginal dryness? Here's what you need to know. ACOG.
Angelou, K., Grigoriadis, T., Diakosavvas, M., Zacharakis, D., & Athanasiou, S. (2020). The Genitourinary syndrome of menopause: An overview of the recent data. Cureus, 12(4), e7586.
Jokar, A., Davari, T., Asadi, N., Ahmadi, F., & Foruhari, S. (2016). Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: A randomized controlled clinical trial. International Journal of Community Based Nursing and Midwifery, 4(1), 69-78.
Nappi, R. E., Kotek, M., Brešt'anský, A., Giordan, N., & Tramentozzi, E. (2020). Effectiveness of hyaluronate-based pessaries in the treatment of vulvovaginal atrophy in postmenopausal women. Climacteric: The Journal of the International Menopause Society, 23(5), 519-524.
Costantino, D., & Guaraldi, C. (2008). Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: An open, non-controlled clinical trial. European review for medical and pharmacological sciences, 12(6), 411-416.
Dos Santos, C. C., Uggioni, M. L., Colonetti, T., Colonetti, L., Grande, A. J., & Da Rosa, M. I. (2021). Hyaluronic acid in postmenopause vaginal atrophy: A systematic review. The Journal of Sexual Medicine, 18(1), 156-166.
Dweck, A., Bernsley, D., Sylla, S., & Komorowski, J. (2022). A national survey on patient satisfaction and use trends with Revaree, a hyaluronic acid suppository, for vaginal dryness. The Journal of Sexual Medicine, 19(8), S26.
Kellogg-Spadt, S., Silva, T., Krychman, M., Weber, S., Dweck, A., Van Dusseldorp, T. A., & Komorowski, J. (2024). Effects of the vaginal suppository Revaree® Plus on women who experience moderate-to-severe vaginal dryness. 2024 Annual Meeting of The Menopause Society, Chicago, Illinois, United States.
Cleveland Clinic. (2024). Dyspareunia (Painful intercourse). Cleveland Clinic.
DiBonaventura, M., Luo, X., Moffatt, M., Bushmakin, A. G., Kumar, M., & Bobula, J. (2015). The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and Western Europe. Journal of women's health (2002), 24(9), 713-722.
Edwards, D., & Panay, N. (2015). Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition? Climacteric, 19(2), 151-161.
Cleveland Clinic. (2022). Vaginal dryness. Cleveland Clinic.
Omidvar, S., Niaki, M. T., Amiri, F. N., & Kheyrkhah, F. (2013). Sexual dysfunction among women with diabetes mellitus in a diabetic center in Amol. Journal of Natural Science, Biology, and Medicine, 4(2), 321-324.
Danesh, M., Hamzehgardeshi, Z., Moosazadeh, M., & Shabani-Asrami, F. (2015). The effect of hysterectomy on women’s sexual function: A narrative review. Medical Archives, 69(6), 387-392.
Krakowsky, Y., Potter, E., Hallarn, J., Monari, B., Wilcox, H., Bauer, G., Ravel, J., & Prodger, J. L. (2022). The effect of gender-affirming medical care on the vaginal and neovaginal microbiomes of transgender and gender-diverse people. Frontiers in Cellular and Infection Microbiology, 11, 769950.
Albornoz, M. A., Burke, J. F., & Threlfall, E. K. (2023). Virgin coconut oil in paste form as treatment for dyspareunia and vaginal dryness in patients with and without rheumatic autoimmune diseases: An efficacy and safety assessment pilot study. Cureus, 15(6), e40501.
U.S. Food and Drug Administration. (2016). 510(k) premarket notification — Repagyn vaginal suppositories. FDA.
U.S. Food and Drug Administration. (2022). 510(k) premarket notification — Revaree Plus vaginal suppositories. FDA.
U.S. Food and Drug Administration. (2017). Learn if a medical device has been cleared by FDA for marketing. FDA.
U.S. Food and Drug Administration. (2021). MAUDE Adverse Event Report — Revaree. FDA.
U.S. Food and Drug Administration. (2022). MAUDE Adverse Event Report — Revaree vaginal suppository. FDA.
Vito, M. D., Mattarelli, P., Modesto, M., Girolamo, A., Ballardini, M., Tamburro, A., Meledandri, M., & Mondello, F. (2015). In vitro activity of tea tree oil vaginal suppositories against Candida spp. and probiotic vaginal microbiota. Phytotherapy Research, 29(10), 1628-1633.
Glazier, E. & Ko, E. (2023). 2002 HRT study comes under criticism. UCLA Health.
Bhupathiraju, S. N., Grodstein, F., Stampfer, M. J., Willett, W. C., Crandall, C. J., Shifren, J., & Manson, J. E. (2018). Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause (New York, N.Y.), 26(6), 603-610.
Cleveland Clinic. (2025). Ospemifene oral tablets. Cleveland Clinic.
Wurz, G. T., Kao, J., & DeGregorio, M. W. (2014). Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clinical Interventions in Aging, 9, 1939-1950.


